SRC
Entrez ID: 6714
Full name: SRC proto-oncogene, non-receptor tyrosine kinase
External links: HGNC UniprotKB Ensembl RefSeq COSMIC
Family: TK: Src: SrcA
Chromosomal location: 20q11.23
Substructure location: Gatekeeper: 341 A-loop: 406...430 G-loop: 276...285 αC-helix: 307...319
Please click HERE
for better display.
SRC
Non-receptor protein tyrosine kinase which is activated following engagement of many different classes of cellular receptors including immune response receptors, integrins and other adhesion receptors, receptor protein tyrosine kinases, G protein-coupled receptors as well as cytokine receptors. Part...
Non-receptor protein tyrosine kinase which is activated following engagement of many different classes of cellular receptors including immune response receptors, integrins and other adhesion receptors, receptor protein tyrosine kinases, G protein-coupled receptors as well as cytokine receptors. Participates in signaling pathways that control a diverse spectrum of biological activities including gene transcription, immune response, cell adhesion, cell cycle progression, apoptosis, migration, and transformation. Due to functional redundancy between members of the SRC kinase family, identification of the specific role of each SRC kinase is very difficult. SRC appears to be one of the primary kinases activated following engagement of receptors and plays a role in the activation of other protein tyrosine kinase (PTK) families. Receptor clustering or dimerization leads to recruitment of SRC to the receptor complexes where it phosphorylates the tyrosine residues within the receptor cytoplasmic domains. Plays an important role in the regulation of cytoskeletal organization through phosphorylation of specific substrates such as AFAP1. Phosphorylation of AFAP1 allows the SRC SH2 domain to bind AFAP1 and to localize to actin filaments. Cytoskeletal reorganization is also controlled through the phosphorylation of cortactin (CTTN) (Probable). When cells adhere via focal adhesions to the extracellular matrix, signals are transmitted by integrins into the cell resulting in tyrosine phosphorylation of a number of focal adhesion proteins, including PTK2/FAK1 and paxillin (PXN) (PubMed:21411625). In addition to phosphorylating focal adhesion proteins, SRC is also active at the sites of cell-cell contact adherens junctions and phosphorylates substrates such as beta-catenin (CTNNB1), delta-catenin (CTNND1), and plakoglobin (JUP). Another type of cell-cell junction, the gap junction, is also a target for SRC, which phosphorylates connexin-43 (GJA1). SRC is implicated in regulation of pre-mRNA-processing and phosphorylates RNA-binding proteins such as KHDRBS1 (Probable). Also plays a role in PDGF-mediated tyrosine phosphorylation of both STAT1 and STAT3, leading to increased DNA binding activity of these transcription factors (By similarity). Involved in the RAS pathway through phosphorylation of RASA1 and RASGRF1 (PubMed:11389730). Plays a role in EGF-mediated calcium-activated chloride channel activation (PubMed:18586953). Required for epidermal growth factor receptor (EGFR) internalization through phosphorylation of clathrin heavy chain (CLTC and CLTCL1) at 'Tyr-1477'. Involved in beta-arrestin (ARRB1 and ARRB2) desensitization through phosphorylation and activation of GRK2, leading to beta-arrestin phosphorylation and internalization. Has a critical role in the stimulation of the CDK20/MAPK3 mitogen-activated protein kinase cascade by epidermal growth factor (Probable). Might be involved not only in mediating the transduction of mitogenic signals at the level of the plasma membrane but also in controlling progression through the cell cycle via interaction with regulatory proteins in the nucleus (PubMed:7853507). Plays an important role in osteoclastic bone resorption in conjunction with PTK2B/PYK2. Both the formation of a SRC-PTK2B/PYK2 complex and SRC kinase activity are necessary for this function. Recruited to activated integrins by PTK2B/PYK2, thereby phosphorylating CBL, which in turn induces the activation and recruitment of phosphatidylinositol 3-kinase to the cell membrane in a signaling pathway that is critical for osteoclast function (PubMed:8755529, PubMed:14585963). Promotes energy production in osteoclasts by activating mitochondrial cytochrome C oxidase (PubMed:12615910). Phosphorylates DDR2 on tyrosine residues, thereby promoting its subsequent autophosphorylation (PubMed:16186108). Phosphorylates RUNX3 and COX2 on tyrosine residues, TNK2 on 'Tyr-284' and CBL on 'Tyr-731' (PubMed:20100835, PubMed:21309750). Enhances DDX58/RIG-I-elicited antiviral signaling (PubMed:19419966). Phosphorylates PDPK1 at 'Tyr-9', 'Tyr-373' and 'Tyr-376' (PubMed:14585963). Phosphorylates BCAR1 at 'Tyr-128' (PubMed:22710723). Phosphorylates CBLC at multiple tyrosine residues, phosphorylation at 'Tyr-341' activates CBLC E3 activity (PubMed:20525694). Involved in anchorage-independent cell growth (PubMed:19307596). Required for podosome formation (By similarity).
View more >>
GO - Biological processes (BP):
activation of protein kinase B activity, adherens junction organization, angiotensin-activated signaling pathway involved in heart process, axon guidance, bone resorption, branching involved in mammary gland duct morphogenesis, cell adhesion, cell-cell adhesion, cell cycle, cell differentiation, cel...
activation of protein kinase B activity, adherens junction organization, angiotensin-activated signaling pathway involved in heart process, axon guidance, bone resorption, branching involved in mammary gland duct morphogenesis, cell adhesion, cell-cell adhesion, cell cycle, cell differentiation, cell population proliferation, cellular response to fatty acid, cellular response to fluid shear stress, cellular response to hydrogen peroxide, cellular response to hypoxia, cellular response to insulin stimulus, cellular response to lipopolysaccharide, cellular response to peptide hormone stimulus, cellular response to platelet-derived growth factor stimulus, cellular response to progesterone stimulus, entry of bacterium into host cell, ephrin receptor signaling pathway, epidermal growth factor receptor signaling pathway, ERBB2 signaling pathway, Fc-gamma receptor signaling pathway involved in phagocytosis, forebrain development, G protein-coupled receptor signaling pathway, integrin-mediated signaling pathway, intracellular signal transduction, leukocyte migration, macroautophagy, negative regulation of anoikis, negative regulation of apoptotic process, negative regulation of cysteine-type endopeptidase activity involved in apoptotic process, negative regulation of extrinsic apoptotic signaling pathway, negative regulation of focal adhesion assembly, negative regulation of intrinsic apoptotic signaling pathway, negative regulation of mitochondrial depolarization, negative regulation of protein-containing complex assembly, negative regulation of telomerase activity, negative regulation of telomere maintenance via telomerase, negative regulation of transcription, DNA-templated, neurotrophin TRK receptor signaling pathway, odontogenesis, oogenesis, osteoclast development, peptidyl-serine phosphorylation, peptidyl-tyrosine autophosphorylation, peptidyl-tyrosine phosphorylation, platelet activation, positive regulation of apoptotic process, positive regulation of canonical Wnt signaling pathway, positive regulation of cyclin-dependent protein serine/threonine kinase activity, positive regulation of cytokine secretion, positive regulation of dephosphorylation, positive regulation of DNA biosynthetic process, positive regulation of epithelial cell migration, positive regulation of ERK1 and ERK2 cascade, positive regulation of glucose metabolic process, positive regulation of insulin receptor signaling pathway, positive regulation of integrin activation, positive regulation of lamellipodium morphogenesis, positive regulation of MAP kinase activity, positive regulation of non-membrane spanning protein tyrosine kinase activity, positive regulation of ovarian follicle development, positive regulation of peptidyl-tyrosine phosphorylation, positive regulation of phosphatidylinositol 3-kinase activity, positive regulation of phosphatidylinositol 3-kinase signaling, positive regulation of platelet-derived growth factor receptor-beta signaling pathway, positive regulation of podosome assembly, positive regulation of protein autophosphorylation, positive regulation of protein kinase B signaling, positive regulation of protein localization to nucleus, positive regulation of protein processing, positive regulation of protein serine/threonine kinase activity, positive regulation of small GTPase mediated signal transduction, positive regulation of smooth muscle cell migration, positive regulation of transcription, DNA-templated, primary ovarian follicle growth, progesterone receptor signaling pathway, protein autophosphorylation, protein destabilization, regulation of bone resorption, regulation of caveolin-mediated endocytosis, regulation of cell-cell adhesion, regulation of cell population proliferation, regulation of cell projection assembly, regulation of early endosome to late endosome transport, regulation of epithelial cell migration, regulation of intracellular estrogen receptor signaling pathway, regulation of postsynaptic neurotransmitter receptor activity, regulation of protein binding, regulation of vascular permeability, response to acidic pH, response to electrical stimulus, response to interleukin-1, response to mechanical stimulus, response to mineralocorticoid, response to nutrient levels, response to virus, signal complex assembly, signal transduction, stimulatory C-type lectin receptor signaling pathway, stress fiber assembly, substrate adhesion-dependent cell spreading, T cell costimulation, transcytosis, transforming growth factor beta receptor signaling pathway, transmembrane receptor protein tyrosine kinase signaling pathway, uterus development, vascular endothelial growth factor receptor signaling pathway, viral process
View more >>
GO - Molecular function (MF):
ATPase binding, ATP binding, BMP receptor binding, cadherin binding, connexin binding, enzyme binding, ephrin receptor binding, estrogen receptor binding, growth factor receptor binding, heme binding, insulin receptor binding, integrin binding, ion channel binding, kinase activity, kinase binding, n...
ATPase binding, ATP binding, BMP receptor binding, cadherin binding, connexin binding, enzyme binding, ephrin receptor binding, estrogen receptor binding, growth factor receptor binding, heme binding, insulin receptor binding, integrin binding, ion channel binding, kinase activity, kinase binding, non-membrane spanning protein tyrosine kinase activity, phosphoprotein binding, protein C-terminus binding, protein kinase activity, protein kinase C binding, protein tyrosine kinase activity, scaffold protein binding, SH2 domain binding, SH3/SH2 adaptor activity, signaling receptor binding, ubiquitin protein ligase binding
View more >>
GO - Cellular component (CC):
actin filament, podosome, cytosol, late endosome, extracellular exosome, lysosome, mitochondrial inner membrane, mitochondrion, nucleus, caveola, extrinsic component of cytoplasmic side of plasma membrane, plasma membrane, ruffle membrane, cell junction, cytoplasm, focal adhesion, glutamatergic syna...
actin filament, podosome, cytosol, late endosome, extracellular exosome, lysosome, mitochondrial inner membrane, mitochondrion, nucleus, caveola, extrinsic component of cytoplasmic side of plasma membrane, plasma membrane, ruffle membrane, cell junction, cytoplasm, focal adhesion, glutamatergic synapse, membrane raft, neuron projection, perinuclear region of cytoplasm, postsynaptic density, postsynaptic specialization, intracellular component
View more >>
SRC proto-oncogene, non-receptor tyrosine kinase
Control panel
Show dataset:
Show substructure:
All sites
Gatekeeper
A-loop
G-loop
αC-helix
Gatekeeper
A-loop
G-loop
αC-helix
Filter mutation sites
All sites
Minimum 5 samples
Minimum 10 samples
Minimum 5 samples
Minimum 10 samples
Data table is loading....
Kaplan plot for SRC in
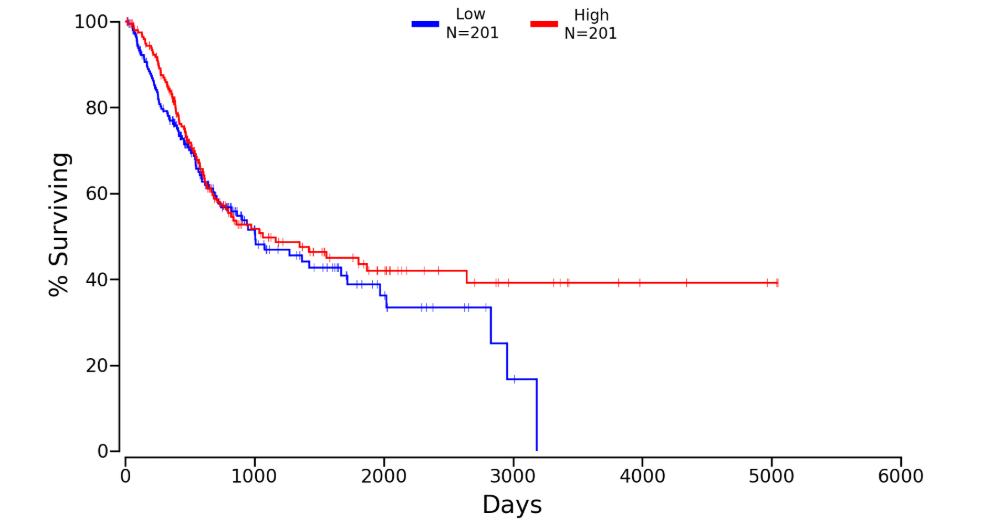
Download patient group data (Lower:Upper = 50%:50%)
*NOTE: the Kaplan plots were collected from OncoLnc