TBK1
Entrez ID: 29110
Full name: TANK binding kinase 1
External links: HGNC UniprotKB Ensembl RefSeq COSMIC
Family: Other: IKK
Chromosomal location: 12q14.2
Substructure location: Gatekeeper: 86 A-loop: 156...178 G-loop: [N/A] αC-helix: 42...46
Please click HERE
for better display.
TBK1
Serine/threonine kinase that plays an essential role in regulating inflammatory responses to foreign agents (PubMed:12692549, PubMed:14703513, PubMed:18583960, PubMed:12702806, PubMed:15367631, PubMed:10581243, PubMed:11839743, PubMed:15485837, PubMed:21138416, PubMed:25636800, PubMed:23453971, PubM...
Serine/threonine kinase that plays an essential role in regulating inflammatory responses to foreign agents (PubMed:12692549, PubMed:14703513, PubMed:18583960, PubMed:12702806, PubMed:15367631, PubMed:10581243, PubMed:11839743, PubMed:15485837, PubMed:21138416, PubMed:25636800, PubMed:23453971, PubMed:23453972, PubMed:23746807, PubMed:26611359). Following activation of toll-like receptors by viral or bacterial components, associates with TRAF3 and TANK and phosphorylates interferon regulatory factors (IRFs) IRF3 and IRF7 as well as DDX3X (PubMed:12692549, PubMed:14703513, PubMed:18583960, PubMed:12702806, PubMed:15367631, PubMed:25636800). This activity allows subsequent homodimerization and nuclear translocation of the IRFs leading to transcriptional activation of pro-inflammatory and antiviral genes including IFNA and IFNB (PubMed:12702806, PubMed:15367631, PubMed:25636800). In order to establish such an antiviral state, TBK1 form several different complexes whose composition depends on the type of cell and cellular stimuli (PubMed:23453971, PubMed:23453972, PubMed:23746807). Plays a key role in IRF3 activation: acts by first phosphorylating innate adapter proteins MAVS, STING1 and TICAM1 on their pLxIS motif, leading to recruitment of IRF3, thereby licensing IRF3 for phosphorylation by TBK1 (PubMed:25636800, PubMed:30842653). Phosphorylated IRF3 dissociates from the adapter proteins, dimerizes, and then enters the nucleus to induce expression of interferons (PubMed:25636800). Thus, several scaffolding molecules including FADD, TRADD, MAVS, AZI2, TANK or TBKBP1/SINTBAD can be recruited to the TBK1-containing-complexes (PubMed:21931631). Under particular conditions, functions as a NF-kappa-B effector by phosphorylating NF-kappa-B inhibitor alpha/NFKBIA, IKBKB or RELA to translocate NF-Kappa-B to the nucleus (PubMed:10783893, PubMed:15489227). Restricts bacterial proliferation by phosphorylating the autophagy receptor OPTN/Optineurin on 'Ser-177', thus enhancing LC3 binding affinity and antibacterial autophagy (PubMed:21617041). Phosphorylates SMCR8 component of the C9orf72-SMCR8 complex, promoting autophagosome maturation (PubMed:27103069). Phosphorylates and activates AKT1 (PubMed:21464307). Seems to play a role in energy balance regulation by sustaining a state of chronic, low-grade inflammation in obesity, wich leads to a negative impact on insulin sensitivity (By similarity). Attenuates retroviral budding by phosphorylating the endosomal sorting complex required for transport-I (ESCRT-I) subunit VPS37C (PubMed:21270402). Phosphorylates Borna disease virus (BDV) P protein (PubMed:16155125). Plays an essential role in the TLR3- and IFN-dependent control of herpes virus HSV-1 and HSV-2 infections in the central nervous system (PubMed:22851595).
View more >>
GO - Biological processes (BP):
cellular response to cytokine stimulus, defense response to Gram-positive bacterium, defense response to virus, dendritic cell proliferation, I-kappaB kinase/NF-kappaB signaling, inflammatory response, innate immune response, negative regulation of gene expression, negative regulation of type I inte...
cellular response to cytokine stimulus, defense response to Gram-positive bacterium, defense response to virus, dendritic cell proliferation, I-kappaB kinase/NF-kappaB signaling, inflammatory response, innate immune response, negative regulation of gene expression, negative regulation of type I interferon production, peptidyl-serine phosphorylation, peptidyl-threonine phosphorylation, positive regulation of I-kappaB kinase/NF-kappaB signaling, positive regulation of interferon-alpha production, positive regulation of interferon-beta biosynthetic process, positive regulation of interferon-beta production, positive regulation of macroautophagy, positive regulation of peptidyl-serine phosphorylation, positive regulation of transcription by RNA polymerase II, positive regulation of type I interferon-mediated signaling pathway, positive regulation of type I interferon production, positive regulation of xenophagy, protein phosphorylation, regulation of neuron death, regulation of type I interferon production, response to virus, TRIF-dependent toll-like receptor signaling pathway, type I interferon production, viral process
View more >>
GO - Molecular function (MF):
ATP binding, identical protein binding, nucleic acid binding, phosphoprotein binding, protein kinase activity, protein phosphatase binding, protein serine/threonine kinase activity...
ATP binding, identical protein binding, nucleic acid binding, phosphoprotein binding, protein kinase activity, protein phosphatase binding, protein serine/threonine kinase activity
View more >>
GO - Cellular component (CC):
cytosol, endosome membrane, nucleoplasm, cytoplasm, intracellular membrane-bounded organelle...
cytosol, endosome membrane, nucleoplasm, cytoplasm, intracellular membrane-bounded organelle
View more >>
TANK binding kinase 1
Control panel
Show dataset:
Show substructure:
All sites
Gatekeeper
A-loop
G-loop
αC-helix
Gatekeeper
A-loop
G-loop
αC-helix
Filter mutation sites
All sites
Minimum 5 samples
Minimum 10 samples
Minimum 5 samples
Minimum 10 samples
Data table is loading....
Kaplan plot for TBK1 in
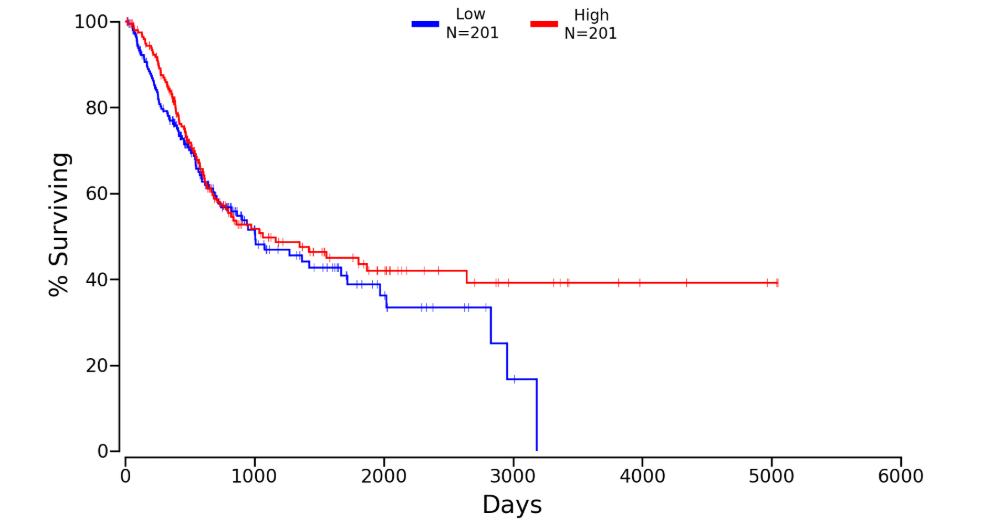
Download patient group data (Lower:Upper = 50%:50%)
*NOTE: the Kaplan plots were collected from OncoLnc